Nomenclature
Short Name:
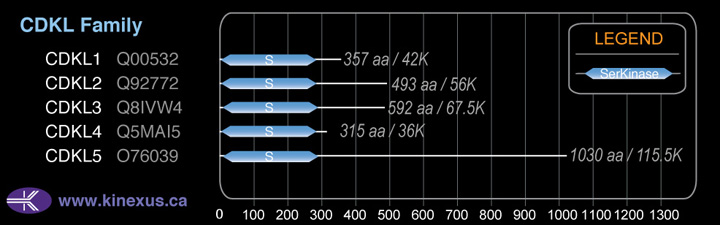
CDKL3
Full Name:
Cyclin-dependent kinase-like 3
Alias:
- Serine/threonine protein kinase NKIAMRE
Classification
Type:
Protein-serine/threonine kinase
Group:
CMGC
Family:
CDKL
SubFamily:
NA
Specific Links
Structure
Mol. Mass (Da):
67,514
# Amino Acids:
592
# mRNA Isoforms:
2
mRNA Isoforms:
67,514 Da (592 AA; Q8IVW4); 51,566 Da (455 AA; Q8IVW4-2)
4D Structure:
NA
1D Structure:
3D Image (rendered using PV Viewer):
PDB ID
Subfamily Alignment
Domain Distribution:
| Start | End | Domain |
|---|---|---|
| 4 | 286 | Pkinase |
Kinexus Products
Click on entries below for direct links to relevant products from Kinexus for this protein kinase.
hiddentext
Post-translation Modifications
For detailed information on phosphorylation of this kinase go to PhosphoNET
Methylated:
K542.
Serine phosphorylated:
S341+.
Threonine phosphorylated:
T158+.
Tyrosine phosphorylated:
Y160+.
Distribution
Based on gene microarray analysis from the NCBI
Human Tissue Distribution
% Max Expression:
Mean Expression:
Number of Samples:
Standard Deviation:
% Max Expression:
Mean Expression:
Number of Samples:
Standard Deviation:
 100
100
1392
34
1044
 1
1
20
16
20
 4
4
56
3
7
 12
12
173
134
648
 36
36
496
42
371
 0.9
0.9
13
64
11
 5
5
75
52
237
 53
53
744
34
1859
 13
13
185
13
233
 2
2
31
104
22
 1
1
16
29
21
 38
38
528
129
563
 2
2
22
17
15
 2
2
29
12
21
 0.9
0.9
12
26
10
 2
2
21
23
23
 1
1
16
320
18
 1
1
20
12
32
 4
4
53
87
44
 28
28
388
138
311
 3
3
38
24
41
 1
1
14
25
16
 2
2
23
5
23
 6
6
85
16
66
 1
1
20
23
22
 64
64
894
82
1698
 2
2
27
21
23
 2
2
26
14
33
 2
2
30
14
35
 6
6
77
42
64
 41
41
566
18
403
 21
21
288
36
414
 33
33
457
116
795
 56
56
776
104
671
 39
39
541
61
1005
Evolution
Species Conservation
PhosphoNET % Identity:
PhosphoNET % Similarity:
Homologene %
Identity:
PhosphoNET % Identity:
PhosphoNET % Similarity:
Homologene %
Identity:
 100
100
100
100 99.6
99.6
99.8
100 98.9
98.9
99.6
99 -
-
-
85 -
-
-
- 67.7
67.7
72.1
87.5 -
-
-
- 80.5
80.5
89.7
81 79
79
87.6
82 -
-
-
- 57.7
57.7
68.9
- 25
25
34.9
- 24.3
24.3
35.6
- 31
31
43
- -
-
-
- 23.4
23.4
35.1
- -
-
-
- -
-
-
- 30.2
30.2
44
- -
-
-
- 22.8
22.8
33.9
- -
-
-
- 23.1
23.1
33.9
- -
-
-
- -
-
-
-
For a wider analysis go to PhosphoNET Evolution in PhosphoNET
Regulation
Activation:
Likely that phosphorylation at Thr-158 and Tyr-160 is required for catalytic activity based on homology with other CDK's.
Inhibition:
Likely that phosphorylation at Tyr-15 is inhibitory based on homology with other CDK's.
Synthesis:
NA
Degradation:
NA
Protein Kinase Specificity
Matrix of observed frequency (%) of amino acids in aligned protein substrate phosphosites
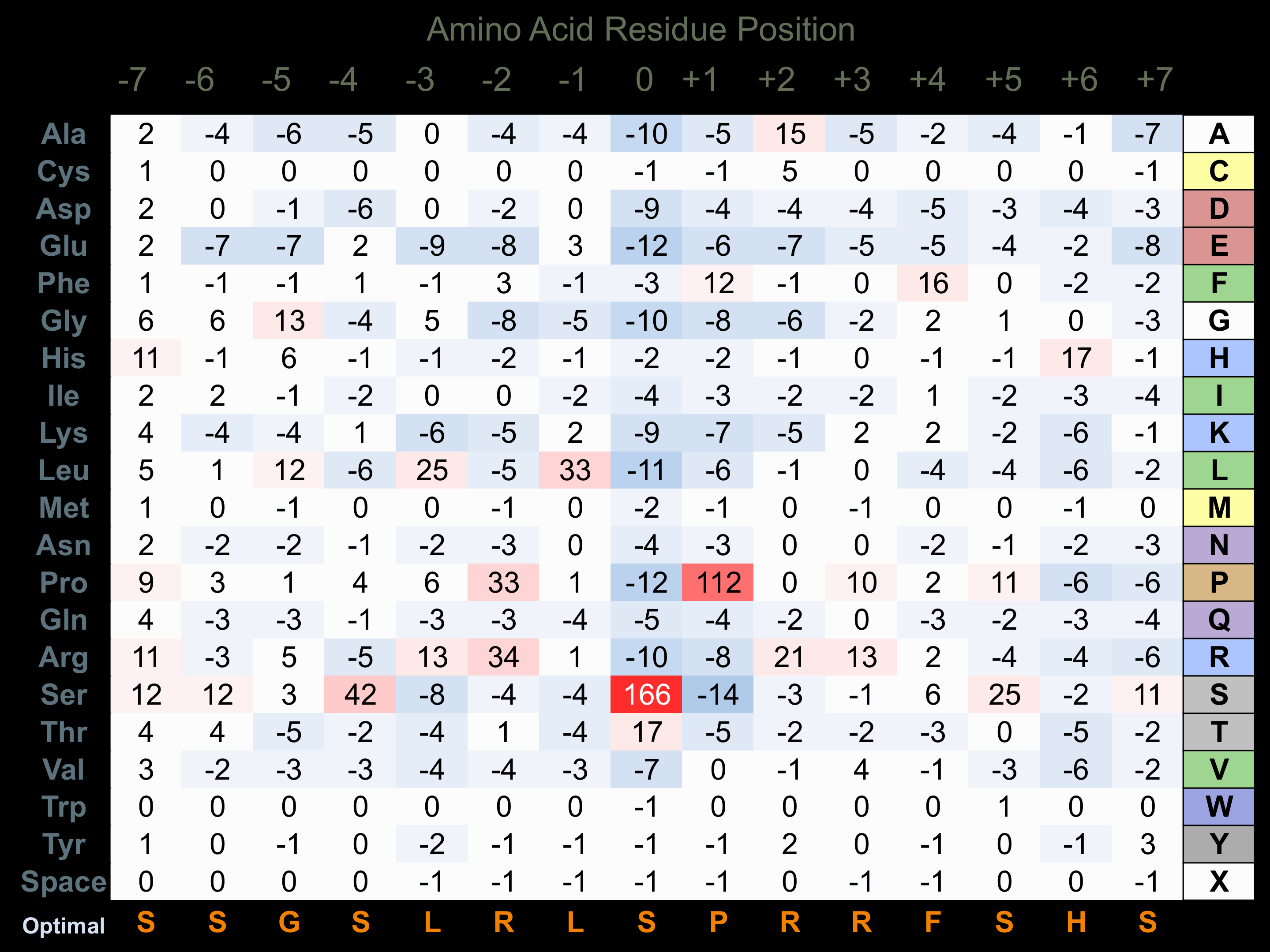
Matrix Type:
Predicted from the application of the Kinexus Kinase Substrate Predictor Version 2.0 algorithm, which was trained with over 10,000 kinase-protein substrate pairs and 8,000 kinase-peptide substrate pairs.
Domain #:
1
Inhibitors
For further details on these inhibitors click on the Compound Name and enter it into DrugKiNET or click on the ID's
Based on in vitro and/or in vivo phosphorylation data
| Compound Name | KD, Ki or IC50 (nM) | PubChem ID | ChEMBL ID | PubMed ID |
|---|
| AST-487 | Kd = 3.9 nM | 11409972 | 574738 | 22037378 |
| Tozasertib | Kd = 350 nM | 5494449 | 572878 | 22037378 |
| Lestaurtinib | Kd = 430 nM | 126565 | 22037378 | |
| Sorafenib | Kd = 490 nM | 216239 | 1336 | 19654408 |
| WZ3146 | Kd > 1 µM | 44607360 | 20033049 | |
| WZ4002 | Kd > 1 µM | 44607530 | 20033049 | |
| A674563 | Kd = 1.1 µM | 11314340 | 379218 | 22037378 |
| AC1NS7CD | Kd = 1.4 µM | 5329665 | 295136 | 22037378 |
| SNS032 | Kd = 1.4 µM | 3025986 | 296468 | 22037378 |
| Foretinib | Kd = 4.4 µM | 42642645 | 1230609 | 22037378 |
Disease Linkage
General Disease Association:
Gene deletion abnormality
Specific Diseases (Non-cancerous):
Chromosome 5q deletion
Gene Expression in Cancers:
The COSMIC website notes an up-regulated expression score for CDKL3 in diverse human cancers of 441, which is close to the average score of 462 for the human protein kinases. The down-regulated expression score of 38 for this protein kinase in human cancers was 0.6-fold of the average score of 60 for the human protein kinases.
Mutagenesis Experiments:
Insertional mutagenesis studies in mice have not yet revealed a role for this protein kinase in mouse cancer oncogenesis.
Mutation Rate in All Cancers:
Percent mutation rates per 100 amino acids length in human cancers: 0.04 % in 24782 diverse cancer specimens. This rate is -53 % lower than the average rate of 0.075 % calculated for human protein kinases in general. Such a low frequency of mutation in human cancers is consistent with this protein kinase playing a role as a tumour requiring protein (TRP).
Mutation Rate in Specific Cancers:
Highest percent mutation rates per 100 amino acids length in human cancers: 0.15 % in 1270 large intestine cancers tested; 0.14 % in 603 endometrium cancers tested; 0.14 % in 589 stomach cancers tested; 0.1 % in 864 skin cancers tested.
Frequency of Mutated Sites:
None > 3 in 19,689 cancer specimens
Comments:
Only 2 deletions, and no insertions or complex mutations are noted on the COSMIC website.

